Clinical research drives forward understanding of health and wellbeing to help people live healthier lives. Research aims to improve treatment, diagnosis, prevention, and healthcare for the benefit of all patients. Here at Tyntesfield, we have an established research team and regularly contribute to local, national, and international studies. We are so grateful to patients who have supported our research in the past and extend our thanks to you all for your contribution to clinical research – it really makes a difference!
Research
How can I take part in a research study?
You can contribute to clinical research by directly taking part in studies or by agreeing to your health information being shared. If you meet the criteria for a study, you may be invited to take part by letter, text or phone call, or during a consultation with the doctor or nurse. You may see a poster on our website, or in the waiting room, and think you may be suitable – please get in touch with our research team to discuss how to volunteer.
Do I have to take part in a research study?
No – taking part in a research study is entirely voluntary and can only be done so with your consent. If you choose to take part in a study, but later change your mind you can withdraw at any stage. You can be completely reassured that this will not affect your ongoing routine care at our practice.
What about my data?
The studies we support all have ethical approval to ensure they are safe for you to take part in and that your information is treated confidentially, in accordance with UK laws and rules (including GDPR). If you consent to be involved in a study, the research team may need us to provide some data from your health records, for example to confirm that you are eligible to take part or to evaluate the new drug or treatment. We would only share identifiable data with your consent.
In some cases, other studies will use ‘informatics tools’ to extract ‘pseudoanonymised’ data from the practice more generally. This means that before it leaves the practice, all patient identifiers (i.e. name, DOB, address or NHS number) are removed so it cannot be identified to a particular patient. You can opt out of your date being used for this purpose here.
Organisations we are currently involved in are:
- CPRD: a government service that provides anonymised patient data for research to improve patient and public health. You cannot be identified from the information sent to CPRD.
- Optimum patient care: a not-for-profit, social enterprise, aimed at improving disagnosis, treatment and management of chronic diseases within primary care.
Who funds the research?
Most research we are involved in are academic studies, hosted by universities. For these studies we receive funding from the National Institute for Health and Care Research (https://www.nihr.ac.uk/). Occasionally we undertake commercial studies and funding for these often comes from pharmaceutical companies. You are welcome to ask any questions you have about the study sponsors.
How do I get in touch?
If you are interested in taking part in any of our studies, or want to know more about research at Tyntesfield, please feel free to give our research team a call on 01275 850611 (direct line) or email us at bnssg.researchtmg@nhs.net.
We look forward to hearing from you!

CPRD
Non-urgent advice: Active Research Studies: Currently Recruiting
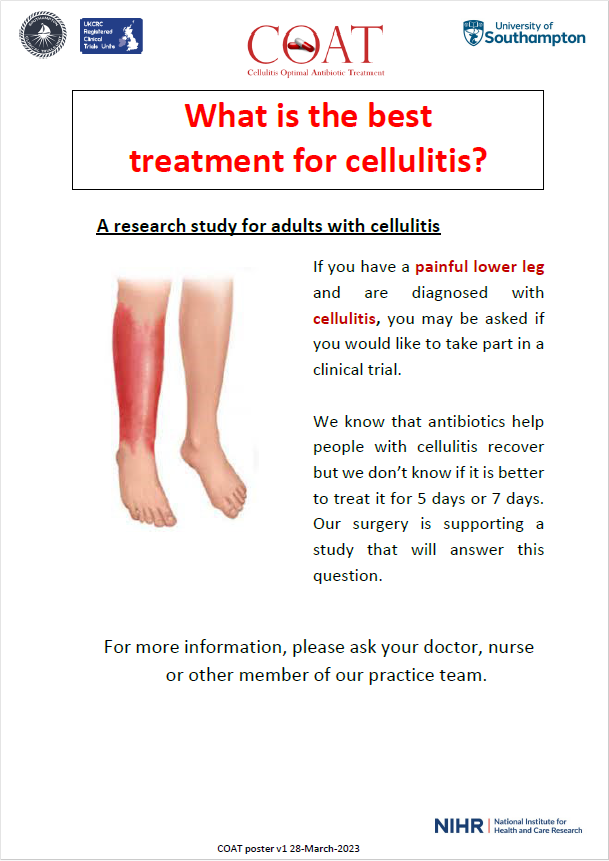
For more information about studies we are currently supporting, please see study posters below.
Orchid
This surveillance study is monitoring immunity of the population to infectious diseases, with a particular focus on respiratory viruses.
Patients attending a routine blood sample appointment will be offered the opportunity to provide an extra sample of blood for inclusion within the study.
Non-urgent advice: Current Research Studies: Closed to Recruitment
For more information about some of the recent studies we have supported, please see below. These studies are now closed to recruitment at Tyntesfield.
ATHENA Shingles Study
Tyntesfield is supporting the ATHENA shingles study. The study wants to find out if taking amitriptyline can prevent the persistent pain that some people get after shingles.
50 years or older and recently got shingles? You may be able to take part. Ask your doctor for more information.
Website: www.bristol.ac.uk/athena-study
Email: athena-study@bristol.ac.uk
Twitter: @AthenaStudy
AvonCAP GP2: General Practice Study about Chest Infections
This study is about chest infections and worsening of asthma, COPD and heart failure. They want to understand: (a) what impact these illnesses have on patients and the NHS; (b) how many of these illnesses could be prevented by vaccination (e.g. with COVID vaccines and other vaccines that are being developed); and (c) what groups of patients might benefit most from vaccination.
This study is led by researchers at the University of Bristol and is funded by Pfizer. For more information, please see the study website or contact the research team by email bnssg.avoncapgp.tmg@nhs.net or phone 0117 958 1405.
Short films: Why take part in research? About the AvonCAP GP2 study
The Attack Study
The ATTACK trial has now finished recruitment. The study aimed to see whether or not patients with chronic kidney disease (CKD) should take aspirin daily to prevent a first heart attack or stroke. It has recruited over 4,500 people with CKD from GP practices across the UK.
Study website: ATTACK Trial | University of Southampton
HPValidate Study
Tyntesfield Medical Centre were selected to take part in an important study to test the use of self-sampling in the NHS Cervical Screening Programme in England.
Self-sampling is a new method that is being considered for the NHS Cervical Screening Programme; this test can be completed at home and if implemented in the programme could increase access to screening for many people. Before self-sampling can be offered as part of routine screening, it needs to be tested to ensure that the results are as accurate as the results from a sample taken by a doctor or nurse.
HPValidate was a study led by Public Health England to compare self-taken samples with samples taken by a doctor or nurse. The study was designed to help the NHS Cervical Screening Programme to decide what device/s could be used for HPV self-sampling in the future.
We extend our thanks to all our patients who offered their time to support this important research. The summary of findings is now available here: UK NSC: HPValidate study findings - GOV.UK.


RAPID Immune Test
SAFER Trial
Thank you to all our patients who supported the SAFER Trial, a research study about screening for atrial fibrillation (irregular heartbeat) to reduce the risk of stroke.
Researchers at the University of Cambridge are aiming to find out if a national screening programme for atrial fibrillation:
- is possible
- will prevent strokes
- is good value for money
Eligible patients aged 70 years and over received an invitation letter from the practice in the post.
For more information about the SAFER Trial please visit www.safer.phpc.cam.ac.uk. Queries about the trial can be made to the research team on 01223 763491 or SAFER@medschl.cam.ac.uk
Non-urgent advice: Completed Research Studies: Published Results
To learn more about the study findings of research we have supported, please see links to publications below.
Psychological impact of the Coronavirus (COVID-19) pandemic and experience : An international survey
The study aimed to understand how COVID affected us and its impact on our day to day lifestyle. The study aimed to find out what is helpful for people during this time and also what may be causing some people to be affected more than others in terms of their wellbeing.
For study findings, please find links to view talks and publications below:
This study aimed to determine if an internet intervention called ‘SupportBack’, provided both with and without guidance from a physiotherapist over the telephone, was effective in reducing the disability lower back pain causes.

SupportBack2 Summary of Findings

This study aimed to explore if using D-mannose (a food supplement) can reduce the number of Urinary Tract Infections (UTIs) experienced by women who have frequent UTIs.

The ANTLER study was a randomised controlled trial comparing maintenance treatment to discontinuation of antidepressant medication. The aim of the study was to investigate the effectiveness of maintenance antidepressant therapy, as compared with discontinuation of treatment.

The Atlantis study assessed the clinical effectiveness of low-dose Amitriptyline (10–30mg) for adults with IBS in primary care.

More details about the study findings can be found in the study publication below.
Non-urgent advice: Primary Care Research Collaboration and Support (PRECAS)
PRECAS is a forum for those involved in the delivery of primary care research within the South West Central region.

PRECAS
Page created: 03 September 2020


